
All rights reserved –
Privacy Policy –
Terms of Use

How important are cathodic protection systems? What role does the purity of anode material play in galvanic corrosion? How often should anodes be replaced for better hull protection against corrosion?
If you seek to receive solid answers to those questions, and discover reasons why your current cathodic protection isn’t working as it should, this article covers both your inquiries. Let’s dive deeper into those questions, and elaborate on the factors affecting cathodic protection of ship hulls.
Corrosion is a natural occurring process by which metals turn into rust when exposed to oxygen. Statistics show that 90% of ship failures are attributed to corrosion. This translates into major-league financial figures, when we actually understand that the total losses from marine transport corrosion ship damage is 3% of the world’s GDP!

The factors that relate to ship corrosion are:
The strictest definition for noble metals refers to the filled electron d-band of the metal, and it’s more of a definition used by chemists. An easier to understand definition claims that a noble metal is one which resists oxidation and corrosion, like platinum, gold and silver. In the opposite direction stand the least noble and more active metals like Magnesium, Zinc and Aluminium.
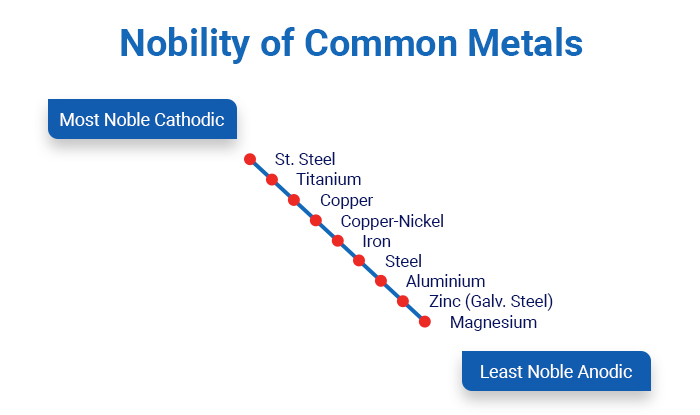
As depicted in the diagram above, Stainless Steel, Titanium and Copper are more noble than Aluminium for example. Vessel hulls should be built with non-corrosive materials and in combination with anodes, they can resist cathodic corrosion and avoid rust.
If we’d like to give a cathodic protection definition, we would have to talk about galvanic corrosion (or else bimetallic corrosion). Galvanic corrosion occurs when two dissimilar metals get in contact with each other when immersed in a conductive solution (in our case water), and one corrodes preferentially to the other. This means that one metal, the cathode, is protected underwater, whilst the other, the anode, is sacrificed (thus sacrificial anode). Ιn a few words, cathodic protection is the protective system applied on ship hulls to protect them against naturally occurring corrosion while they are submerged underwater.
Corrosion is an irresistible process of natural metal destruction that occurs when some metals are exposed to the environment. The metal will start converting into its more stable forms, such as iron III oxide or hydroxide, widely known as rust. When it comes to on-ground steel establishments for example, rust is caused by the oxidation reaction between the atmosphere’s oxygen and moisture that comes into contact with metallic surfaces.
The same happens for metals underwater. If no protective anodes are used, corrosion will eventually deform the ship’s hull, and the ship will need to undergo offshore maintenance shortly after the oxidation’s effect is visible to the eye. However, using protective anodes placed on the hull, the protected cathode (hull) will remain intact while the anodes succumb themselves to corrosion.
Cathodic protection systems using sacrificial anodes are set to supply electrons to the exposed metal delivering a cathodic current. To prevent corrosion at the cathode, the minimum number of electrons that need to be supplied is equal to the number of electrons involved in the oxidation process.
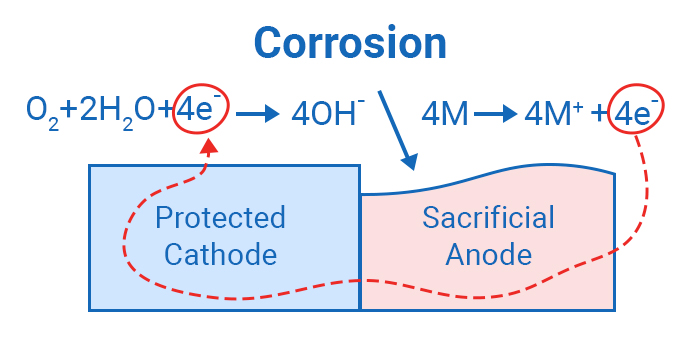
The anode corrosion process- Alt text Cathodic protection system with sacrificial anode
The image above shows how cathodic protection actually works. Electrons travel from the anode through the cathode, and then they get captured by the water particles to form hydroxide. The anode gradually wears away after losing a substantial amount of electrons, and will require replacement for continuous protection of the cathode.
So what is a cathodic protection system? It’s a system that protects a ship from corrosion, usually with the help of sacrificial anodes. How efficient is this system? Sacrificial anode cathodic protection systems efficiency is affected by five major factors which are possible to control and overwatch. These factors are:
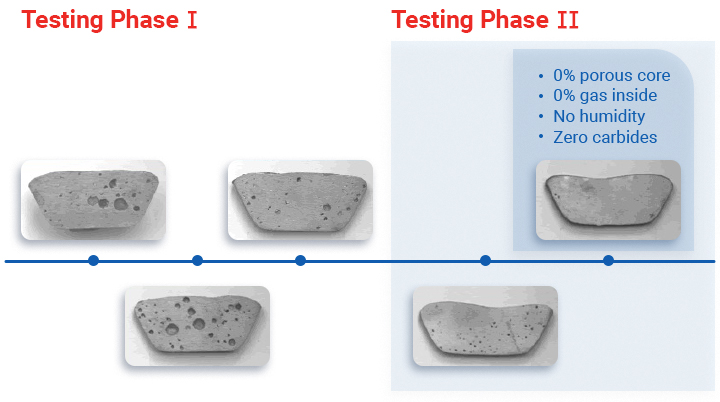
The metal purity used to manufacture sacrificial anodes for cathodic protection systems is highly important. The homogeneity of a material determines the flow of the oxidation process, and if there are contaminants within the metal, it might prove to be harmful to your vessel.
Purity of raw materials means:
When there are pores, layers, impurities or other additives (paint, oxides or iron) in the alloy, there is a higher possibility the anode will be unstable and have lower performance.
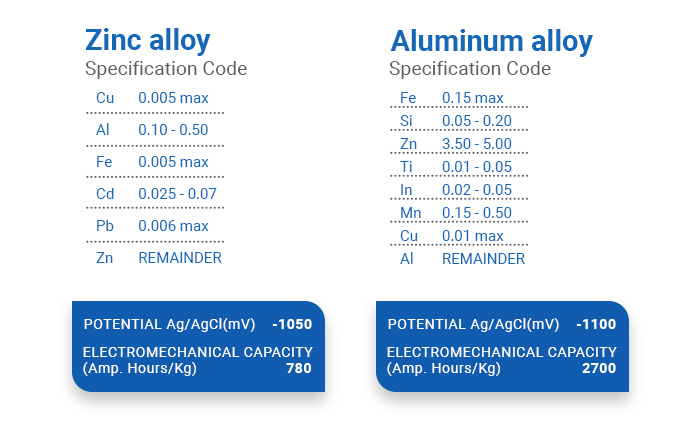
Another factor to consider before buying sacrificial anodes is the alloy type. It is known that some metals are more suitable for sea waters while others offer protection for fresh or even brackish waters.
Let’s see which metals are suitable for alloys meant for cathodic protection systems with anodes:
As to how anodes should be installed, a well-trained personnel is the only option here. Installation directives should not be ignored since some crucial details could be reasons a ship undergoes corrosion decay, despite the fact that there have been sacrificial anodes in place!
If anodes have not been properly installed, your ship’s hull may still suffer corrosion damage over time.
The installation surface has to go through a pre-treatment phase, and weldings have to follow the manufacturer’s instructions. Installers are strongly advised to avoid applying any paint on anodes and follow the installation guide design. We will talk about this later in this article.
Now let’s talk about the “time” factor. Every ship owner or captain or even ship’s procurement manager has to consider this factor when managing marine supplies. The question remains:
When should you replace your ship’s anodes to maintain a cost-effective plan?
If anodes are replaced more often than they should, you could be spending more than necessary to maintain the ship in great condition. On the other hand, if you don’t replace anodes on time, you spend more on the long run since the ship will require massive maintenance, not to mention the cost of having a ship ashore for months.
Katradis S.A. team can calculate anode lifetime and consult you on matters of their replacement. All our anodes get into testing and carry their own unique ID production line marking, allowing us to measure their lifespan and assess the replacement timeframe.
We know our materials. We can foresee anode behaviour according to your ship.
Thus, we are able to calculate the maximum timespan your ship is 100% cathodically protected by our anodes.

By trusting Katradis S.A. for your cathodic protection, you can be certain about cost-effective and on time sacrificial anode replacement.
Besides using top quality pure materials and organising cost-effective replacements, there is something else you need to consider for your cathodic protection systems. The cathodic protection design plays an equally important role here, since it is actually the installation blueprint ensuring the optimum placing and fitting of the anodes, so that your ship is undoubtedly protected from corrosion.
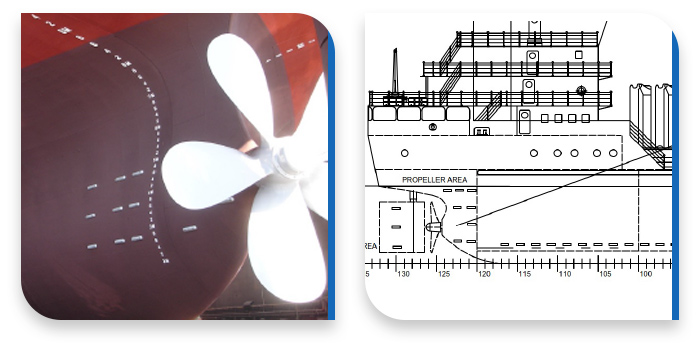
A metallic materials expert will conduct a study on your ship and will create the blueprint of anode installation for you. Following the instructions of this design keeps your hull and propeller cathodically protected, so that your ship can travel overseas for longer periods of time.
Katradis S.A. is a primary raw material certificate holder and maintains a continual quality control process as a manufacturer of sacrificial anodes and cathodic protection systems designer. With highly experienced associates and a high-end spectrometer for testing, our research and development teams strive to find the best solutions applicable to maritime and the shipping industry. Our production procedure follows our strict quality policy to ensure effectiveness and suitability.
Follow us on Facebook, Instagram and LinkedIn. Keep in touch with us to discover the best and safest solutions for your crew, ship and marine operations.